Sucroflo®~ an option for compressible sugar fine granular with good flowing ability
The definition of sucrose
Sucrose is a typical sweetener and a benchmark for sweetness assessment. It is a renewable natural resource and a green food. It has taste and processing properties that other synthetic sweeteners cannot match. Also, research on its physiological properties has also shown that sucrose is an essential nutrient for the human body and the ideal sweetening aid.
Sucrose is a disaccharide, which is formed by the condensation of one molecule of glucose and one molecule of fructose. It is widely found in the stems, leaves, fruits and seeds of plants. It is most abundant in beets and sugar cane, both beets or sugar cane have become the main raw materials for producing sucrose. The white sugar, brown sugar and granulated sugar we use regularly are derived from sucrose. Pure sucrose is a white crystal, easily soluble in water, with a melting point of 185-186°C. When heated to 200°C, it turns into caramel. People often use this property to heat white sugar into caramel to color food during cooking.
01 The application of sucrose in pharmaceutical preparations
Based on the above characteristics, sucrose is widely used in the production of food, medicine, and health products. In the field of the pharmaceutical industry, it can be used as a nutritional sweetener, excipient, filler, adhesive, diluent, pore-forming agent, pellet core, freeze-drying protective agent, etc. There are many varieties of pharma-grade sucrose on the market.
Under the growing demand from the market, we have developed a special pharma-grade sucrose with fast-dissolving properties which can be used for direct compression of tablets.
Product name: Sucroflo®
Chinese name: Sugar Fine Granular
.png)
Definition of compressible sucrose in the current edition of the Chinese Pharmacopoeia
Compressible sucrose is made from co-crystallization of sucrose and other excipients, such as maltodextrin.
It can also be made by dry granulation process.
The final dry powder should contain 95.0% - 98.0% of sucrose (C12H22O11)
This product may contain starch, maltodextrin, inverted sugar and appropriate amount of residual flow aids.
Reference from: Part IV of the Chinese Pharmacopoeia, 2020 edition
Sucroflo® Sugar Fine Granular is made from ordinary white cane/beet sugar and is processed through special techniques to change the physical properties of sucrose. The sucrose becomes suitable for direct compressing with higher purity (99%) than traditional compressible sucrose.
02 Features of Sucroflo®
Sucroflo® is a fast-dissolving pharma-grade sugar fine granular for direct compression tabletting with minimal risk of discoloration and is not prone to hygroscopicity and hardening during long-term storage.
.png)
.png)
Feature 1: Originated from European technology, creating superior tabletting performance
Sucroflo® is developed by Tianjin Convinced & Condar Pharmaceutical Co., Ltd. and a European research centre. It has been trial and pilot-tested multiple times in Ireland, the UK and China. The R&D process lasted two and a half years. Its excellent physical indicators enhance the tabletting quality of the preparation.
1.1 Fluidity test and comparison
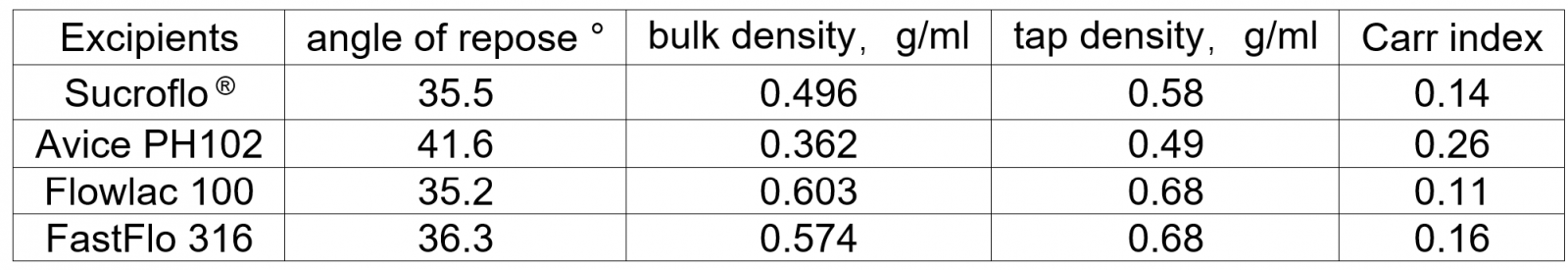
In the process of direct compression tabletting, the excipients must have good flowability and excellent compressibility. To verify the tabletting performance of this product, we conducted relevant research and testing on its powder properties and compared it with several commonly used direct compression excipients.
The main indicators for evaluating powder flowability are angle of repose, bulk density, tapped density, Carr index, etc. It is generally believed that the material with an angle of repose <40°, bulk density >0.4g/ml, and Carr index <0.3 can meet the needs of powder flowability for tabletting production. The comparison results are as follows (see Table 1):
Sucroflo® Sugar Fine Granular compared to several commercially available direct compression excipients
Table 1 - Powder fluidity index test results
/Public/userfiles/files/Liquidity Test Video.mp4
(Click the link to view the liquidity test video)
1.2 Tablet Compression Testing
Take several commonly used direct compression excipients and add 1% magnesium stearate to each of them, mix well and compress into tablets (tablet diameter 12.5mm). The hardness of the tablets pressed by the tablet press under the same pressure (5T) is used as the evaluation index for inspection, and abnormal conditions such as tablets cracking and cap removal are also inspected. The test results are as follows (see Table 2):
Table 2 - Comparison of Sucroflo® Sugar Fine Granular with several commonly used direct compression excipients on the market
.png)
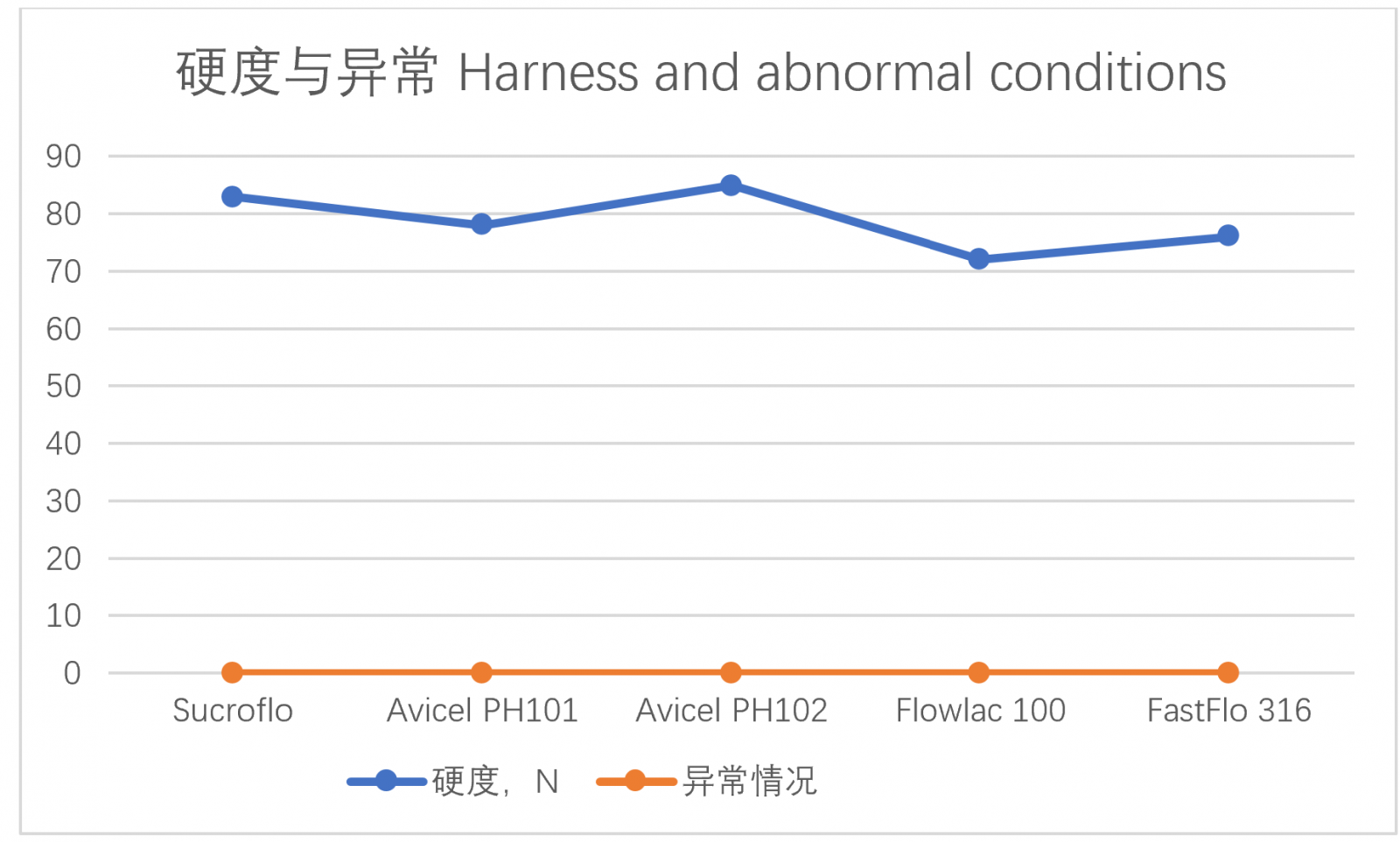
As shown in Table 2, Sucroflo® Sugar Fine Granular showed good compressibility and formability during direct tablet compressing. The tabletting performance was comparable to or better than that of several commonly used imported direct compression excipients.
Feature 2: Instant dissolution
Sucrose is highly soluble in water (1 ml of water can dissolve about 2 g of sucrose at room temperature), but its dissolution speed is not fast.
The production process of Sucroflo® Sugar Fine Granular transforms sucrose from crystals to loose granulated form with more pores, which facilitates instant dissolution.
Take the same weight of white sugar and Sucroflo® Sugar Fine Granular add same amount of water at room temperature, and shake gently. It was observed that Sucroflo® Sugar Fine Granular almost completely dissolved in 3 seconds;
Add 5g of Sucroflo® Sugar Fine Granular and spray-dried mannitol (Pearlitol 200SD) into 150g of pure water at 20 degrees Celsius, simultaneously without shaking, and record the solubility of the two in water.
/Public/userfiles/files/Solubility Test Video.mp4
(Click the link to view the solubility test video)
Sucroflo® Sugar Fine Granular has a fast dissolution property, which can accelerate tablet disintegration in production and increase the drug release rate. With this property, this product can also replace traditional sugar cubes, which can be used in instant drinks and is widely used in other food productions.
.png)
Feature 3: Extremely low risk of discoloration
Have you noticed the white sugar at home will slowly turn yellow after being left for a long time? This is usually caused by the sulfite method used in sucrose production; this production process is relatively low in cost, but the impurity content is relatively high. The sucrose produced by this process has a risk of discoloration during the drug production process - especially common on sugar-coated tablets. To eliminate the risk, we thoroughly screen the manufacturers of starting materials, select high-quality raw materials, and customize special processes to ensure that the end product has better storage stability.
.png)
Feature 4: Less moisture absorbing and hardening during long-term storage
Sucroflo® Sugar Fine Granular contains very low moisture, and the anti-hygroscopic inner and outer packaging design makes our products able to keep out moisture and clumping during long-term storage. It perfectly meets the needs of high-end customers for product stability.
.png)
Sucroflo® Sugar Fine Granular CDE registration status
Sucroflo® Sugar Fine Granular was registered on CDE in 2021, registration number: F20210000469.
03 Tianjin Convinced & Condar Pharmaceutical Co., Ltd.,

Tianjin Convinced & Condar Pharmaceutical Co., Ltd., established in 2015, is a joint venture between Beijing Fengli Jingqiu and Jingliang Biotech Group. Jingliang Biotech is a subsidiary of Beijing Capital Agribusiness & Foods Group, a state-owned group with an excellent brand image and influence.
Tianjin Convinced & Condar mainly engages in the research, production, and sales of customised pharmaceutical excipients, with application laboratories and drug development and analysis laboratories capacity. Covering an area of 8.2 acres, the production site strictly adheres to the GMP system.
Our mission is to explore new functional excipients and provide essential customized products and services to elevate the pharmaceutical industry.
Our Positioning:
1) To satisfy the requirements of special pharmaceutical excipients required for consistency evaluation;
2) To satisfy the requirements of excipients with applicable quality but not meeting the local regulations;
3) To satisfy the demand of special excipients in IND and ANDA market;
4) To satisfy the demand of the production of small varieties pharmaceutical excipients and shortage drugs;
5) To simplify the development and production of specific functional excipients in the pharmaceutical process.
.jpg)
©2022Beijing Fengli Jingqiu Pharmaceutical Co., Ltd. All rights reserved.

